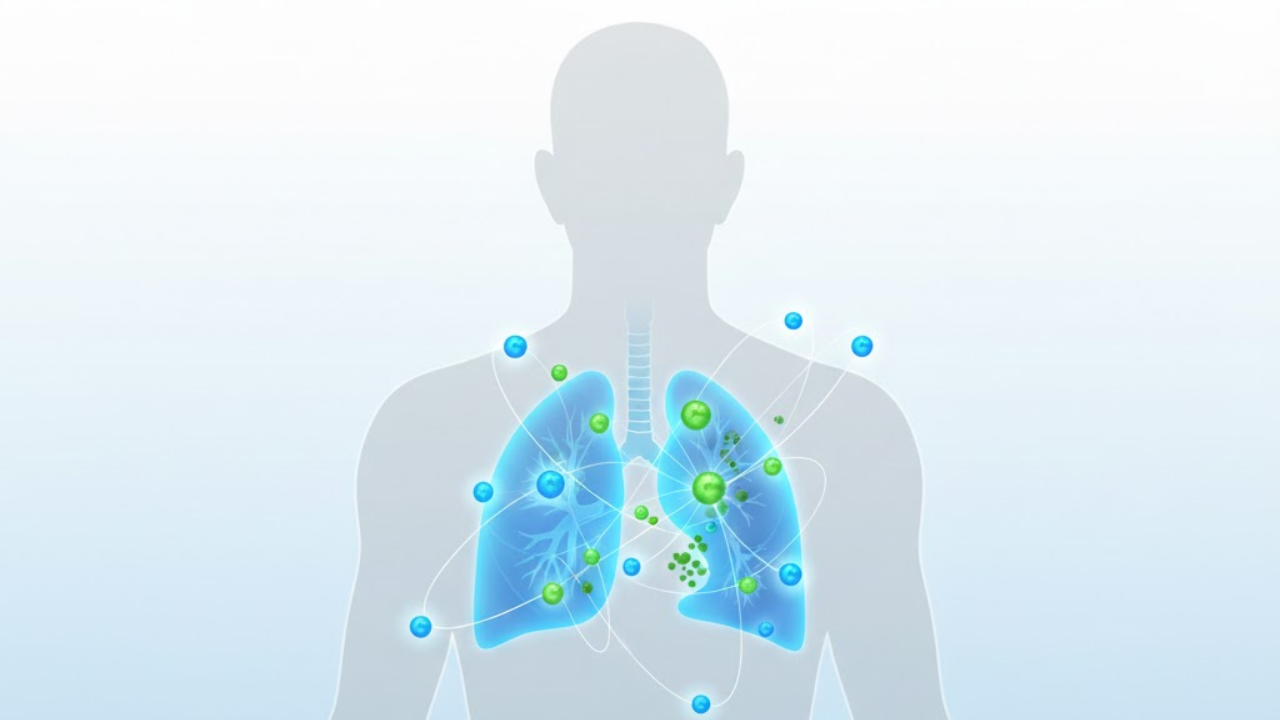
Anktiva, also known as nogapendekin alfa inbakicept, is a new cancer therapy that boosts the immune system to target cancer cells. The Saudi Food and Drug Authority has become the first regulatory body globally to grant conditional approval for Anktiva in advanced non-small cell lung cancer and high-risk bladder cancer, offering a new treatment option for patients with limited alternatives.
Trending
- Indore water contamination: Rahul Gandhi visits families of deceased
- ‘Help them refresh’: Shiv Sena shifts newly elected corporators to Bandra luxury hotel; Mahayuti eyes mayor post
- Big relief for Punjab’s farmers as Centre has agreed to shift border fence closer to IB: CM Mann
- BMC polls setback: Ex-Mumbai Congress president seeks Varsha Gaikwad’s resignation; party issues show-cause notice
- ‘People haven’t rejected Ajit dada, rather … ‘: Fadnavis on Pune win; hails leadership of PM Modi
- ‘We don’t sell achievements’: Indian-origin owner of Arizona’s ‘profile building’ service on row over ‘pay and get EB-1A Green Card’
- Trumpiana: No pause for the petulant prize fighter
- ChatGPT Go goes global: OpenAI makes AI affordable; expands India-exclusive subscription